Mensaje de error
Warning: Array to string conversion in Drupal\deltalabdata\Controller\AgrupacioController->showDetails() (line 149 of modules/custom/deltalabdata/src/Controller/AgrupacioController.php).
Drupal\deltalabdata\Controller\AgrupacioController->showDetails() call_user_func_array() (Line: 123) Drupal\Core\EventSubscriber\EarlyRenderingControllerWrapperSubscriber->Drupal\Core\EventSubscriber\{closure}() (Line: 638) Drupal\Core\Render\Renderer->executeInRenderContext() (Line: 121) Drupal\Core\EventSubscriber\EarlyRenderingControllerWrapperSubscriber->wrapControllerExecutionInRenderContext() (Line: 97) Drupal\Core\EventSubscriber\EarlyRenderingControllerWrapperSubscriber->Drupal\Core\EventSubscriber\{closure}() (Line: 181) Symfony\Component\HttpKernel\HttpKernel->handleRaw() (Line: 76) Symfony\Component\HttpKernel\HttpKernel->handle() (Line: 53) Drupal\Core\StackMiddleware\Session->handle() (Line: 48) Drupal\Core\StackMiddleware\KernelPreHandle->handle() (Line: 28) Drupal\Core\StackMiddleware\ContentLength->handle() (Line: 32) Drupal\big_pipe\StackMiddleware\ContentLength->handle() (Line: 201) Drupal\page_cache\StackMiddleware\PageCache->fetch() (Line: 138) Drupal\page_cache\StackMiddleware\PageCache->lookup() (Line: 87) Drupal\page_cache\StackMiddleware\PageCache->handle() (Line: 48) Drupal\Core\StackMiddleware\ReverseProxyMiddleware->handle() (Line: 51) Drupal\Core\StackMiddleware\NegotiationMiddleware->handle() (Line: 36) Drupal\Core\StackMiddleware\AjaxPageState->handle() (Line: 51) Drupal\Core\StackMiddleware\StackedHttpKernel->handle() (Line: 741) Drupal\Core\DrupalKernel->handle() (Line: 19)Warning: Array to string conversion in Drupal\deltalabdata\Controller\AgrupacioController->showDetails() (line 149 of modules/custom/deltalabdata/src/Controller/AgrupacioController.php).
Drupal\deltalabdata\Controller\AgrupacioController->showDetails() call_user_func_array() (Line: 123) Drupal\Core\EventSubscriber\EarlyRenderingControllerWrapperSubscriber->Drupal\Core\EventSubscriber\{closure}() (Line: 638) Drupal\Core\Render\Renderer->executeInRenderContext() (Line: 121) Drupal\Core\EventSubscriber\EarlyRenderingControllerWrapperSubscriber->wrapControllerExecutionInRenderContext() (Line: 97) Drupal\Core\EventSubscriber\EarlyRenderingControllerWrapperSubscriber->Drupal\Core\EventSubscriber\{closure}() (Line: 181) Symfony\Component\HttpKernel\HttpKernel->handleRaw() (Line: 76) Symfony\Component\HttpKernel\HttpKernel->handle() (Line: 53) Drupal\Core\StackMiddleware\Session->handle() (Line: 48) Drupal\Core\StackMiddleware\KernelPreHandle->handle() (Line: 28) Drupal\Core\StackMiddleware\ContentLength->handle() (Line: 32) Drupal\big_pipe\StackMiddleware\ContentLength->handle() (Line: 201) Drupal\page_cache\StackMiddleware\PageCache->fetch() (Line: 138) Drupal\page_cache\StackMiddleware\PageCache->lookup() (Line: 87) Drupal\page_cache\StackMiddleware\PageCache->handle() (Line: 48) Drupal\Core\StackMiddleware\ReverseProxyMiddleware->handle() (Line: 51) Drupal\Core\StackMiddleware\NegotiationMiddleware->handle() (Line: 36) Drupal\Core\StackMiddleware\AjaxPageState->handle() (Line: 51) Drupal\Core\StackMiddleware\StackedHttpKernel->handle() (Line: 741) Drupal\Core\DrupalKernel->handle() (Line: 19)
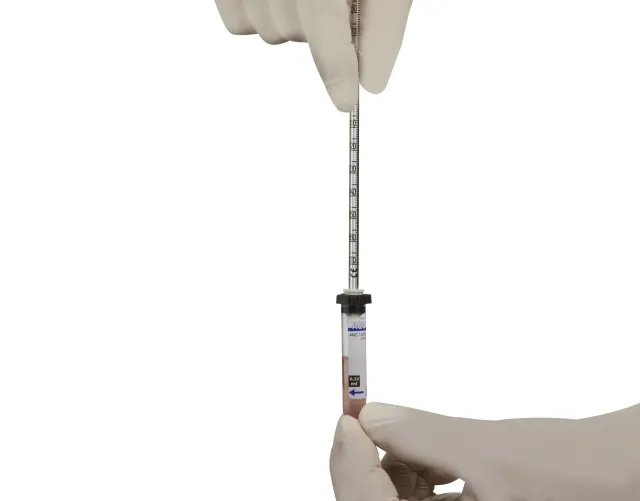
Velocidad de sedimentación: sistema micro (para pediatría)
The Micro system for the determination of the erythrocyte sedimentation rate (ESR) is a method that allows the use of minimum volumes of blood. Ideal for all types of tests, this set is especially recommended for Paediatrics and for all laboratories working with small volumes of blood. The results obtained are fully comparable to those obtained by using the standard Westergren method (Macro). The set consists of a tube filled with 0.08 ml of Tri-sodic Citrate 0.106M for 0.32 ml of blood, according to the standard Westergren method, and one pipette of pressure filling (inner diameter 1.25 mm). The pipette is made of PS and displays graduations in 1 mm increments up to 180 mm.
PACKAGING: Each case is 20 x 40 x 37 cm (length x width x height) and contains:
•4 expanded polystyrene racks of 100 tubes each. •4 boxes of 100 pipettes each (vertically positioned). TUBE FEATURES: •Ultra clear polypropylene tube, highly resistant to shocks. •Length: 54.5 mm, 56 mm with cap. Inner diameter: 10 mm. Outer diameter: 11.3 mm.
•Very flexible rubber cap featuring two cross cuts, specially designed to reseal automatically after the introduction or withdrawal of the sample. This system ELIMINATES THE NEED TO REMOVE THE STOPPER WHEN INTRODUCING OR WITHDRAWING THE SAMPLE.
•Material: thermoplastic rubber. Colour: black (inorganic dye). Max. diameter: 15.4 mm. Length of a cut: 7 mm. Max. useful diameter: 6 mm.
•The tube label includes the Eurotubo® brand, description/code, batch number/expiry date and filling line at 0.32 ml.
Label dimensions: 18 x 35 mm.
Activity: blood anticoagulant by precipitating the ion Ca++ of the blood, thus avoiding its coagulation.
Method: the method consists in carrying out the readings at 50 and 100 minutes while pipettes stand in a vertical position. This can be achieved either by reading directly the pipette graduation or by means of automatic devices after setting the required time. The clinical results thus obtained are fully comparable to those obtained with the standard Westergren method.
Objective: determination of the erythrocyte sedimentation rate (ESR) by the Westergren method.
Ratio: 1 volume of citrate for 4 volumes of blood (following the standards recommended by the Committee for Standardisation of Haematology according to the Westergren method).
Stability: guaranteed 12 months from manufacturing date. Storage: avoid long exposure to the sun.
It is recommended to store at tempreatures below 45ºC IN COMPLIANCE WITH: Directive 98/79/CE. “In vitro” diagnostic medical devices. Standards related with the Quality System o with the product:
•UNE EN ISO 9001 Quality Management System
•UNE EN ISO 13485 Medical Devices - Quality Management Systems
•UNE EN ISO 14971 Risk Management of Medical Devices
•UNE EN ISO 6710 Single use tubes for blood sampling
•UNE EN ISO 15223-1 Medical devices. Symbols used on labels, labeling and information to be supplied. Part 1: General requirements.
•UNE EN ISO 13079 Laboratory glass and plastics ware -- Tubes for the measurement of the erythrocyte sedimentation rate by the Westergren method